Bioactive Compounds in Virgin Olive Oil of the PDO Montoro‐Adamuz
We just received a preview article to be published in the prestigious scientific journal JAOCS on research results in extra virgin olive oils of the Designation of Origin Montoro – Adamuz corresponding to the last seven seasons. Here is the whole contents article Drs C. Romero, Maria. V. Ruiz-Mendez and Manuel Brenes and their conclusions show that the line of work we follow is right, seeking to create the healthiest AOVE.
C. Romero1 · Mª. V. Ruiz‑Méndez1 · Manuel Brenes1
Received: 24 March 2015 / Revised: 22 January 2016 / Accepted: 4 February 2016
Abstract
Virgin olive oil (VOO) is generally recognized as a healthy fat because of its fatty acid composition and content in minor compounds but a wide range of these substances can be found in commercial oils. The concentration of compounds with attributed health benefits were analyzed in VOO of the PDO Montoro-Adamuz. Oleic acid represented around 79 % of the total fatty acids, and the mean squalene and tocopherols concentrations were 5800 and 247 mg/kg respectively. Despite the changes found in polyphenols concentration in the oils analyzed for six consecutive crops, these substances accounted for more than 700 mg/kg. Moreover, the effect of irrigation regime and sun radiation on the content in bioactive substances of these oils was also assessed. No signifcant differences were detected between oils from trees irrigated ad libitum or rain-feed. In contrast, the level of tree radiation exerted a great effect on the concentration of bioactive substances in oils. Oils from trees cultivated in a sunny area (south orientation) had a higher percentage of oleic acid and concentration in phenolic compounds than those from shady areas (north orientation). The opposite was detected for tocopherols and squalene which were more concentrated in oils from olives of the shady area. The results obtained in this study point out VOO of the PDO Montoro-Adamuz as a very healthy fat due to their composition in bioactive substances, in particular their richness in phenolic compounds.
Introduction
The benefits of consuming olive oil have been known since antiquity and were traditionally attributed to its high con- tent of oleic acid. However, olive oil possesses a myriad of biologically active minor components such as tocopherols, sterols, squalene and particularly phenolic compounds that make this fat one of the healthiest among vegetable oils worldwide.
The positive effect of dietary monounsaturated fats in preventing cardiovascular diseases and cancer has exten- sively been reported [1, 2], and claimed by international food and health agencies. The US Food and Drug Administration has stated that the consumption of olive oil may reduce the risk of coronary heart disease due to its content in monounsaturated fat [3], and the European Food Safety Authority has reported that “replacing saturated fats in the diet with unsaturated fats contribute to the maintenance of normal blood cholesterol level” [4].
Sterols and squalene are other bioactive substances pre- sent in olive oil. Phytosterols or plant sterols can reduce intestinal absorption of cholesterol and subsequently serum cholesterol levels thereby they can contribute to prevention of myocardial infarction [5]. They have also been attributed with anticarcinogenic and antitumor properties [6].
Moreover, α-tocopherol, which is the most common form of vitaminm E, is the major tocopherol in olive oil, and it shows the highest antioxidant and biological activ- ity among tocopherols and tocotrienols [7]. The EFSA has also indicated that vitamin E “contributes to the protection of cells from oxidative stress” [4].
Of particular interest from a nutritional point of view the phenolic compounds are that they comprise a high number of substances found at levels of mg/kg oil but recognized with many nutritional activities because they exhibit protective effects against neurodegenerative and cardiovascular diseases [8]. Precisely, the European Commission has recently approved a health claim for olive oil polyphenols because of their contribution to the protection of LDL particles from oxidative damage [4].
There are many other minor substances in olive oil (carotenoids, aliphatic alcohols, triterpenic acids and oth- ers) although due to their low content in oil and/or low biological activity the scientific community has paid them less attention up to now.
The chemical composition of virgin olive oil (VOO) is in uenced by many variables like olive cultivar, agronomic and pedo-climatic conditions, fruit maturity and technological factors, among others. Consequently, VOO is not a uniform product but oils with a wide range in chemical composition and content in nutritional substances are commercialized. Hence, consumers are demanding VOO rich in these bioactive substances.
In the last few years, there has been increasing interest in the geographical characterization of VOO according to regulations on Protection Designation of Origin (PDO) and Protected Geographical Indication (PGI) [9]. These regulations are a guarantee of a precise geographical ori- gin that determines sensory and chemical characteristics of oil but content in bioactive substances are not generally certified.
The VOO of the PDO Montoro-Adamuz is obtained from fruits of the Picual (>90 %) and Nevadillo Negro (700 mg/kg oil) [10]. However, no references can be found regarding the amount of these substances and others bioactive in the literature, particularly during several years. The chemical characterization of nutritional substances present in VOO of the PDO Montoro-Adamuz was studied for the first time.
The agricultural area with the denomination Montoro- Adamuz comprises mountainous orchards that suffer extreme Mediterranean climatic conditions, particularly shortage of water in summer and autumn. Hence, the main agronomic factors that can influence the content of these oils in bioactive substances are the irrigation regime and the orientation of the olive trees in the mountain, either in a shady or a sunny area.
Materials and Methods
VOO of the PDO Montoro‐Adamuz
Samples of oil were taken from industrial tanks by authorized personnel of the PDO, and sent to the laboratory for analysis without any stored period. These oils were collected from the season 2008/2009 to 2013/2014, and graded as extra virgin olive oils.
Effect of Irrigation and Sun Radiation on Bioactive Compounds in Oils
Olives were taken from trees located in the geographical area of the PDO Montoro-Adamuz (Córdoba, Spain) during the season 2010/2011 ( first fortnight of December). Fruits of the Picual and Nevadillo negro cultivars were harvested from trees irrigated ad libitum or rain-feed only. All trees were cultivated in the same area by the same grower. Representative fruits (3 kg sample) at a maturity stage at which 50 % of the fruits displayed black color on the surface, and the rest of them had yellow color were hand-picked from 6 trees of each assay and brought to the laboratory for oil extraction the same day.
Fruits of both cultivars were also hand-picked from another different orchard from trees located in either a shady or a sunny area. In the northern hemisphere, the area of the mountain facing south receives more solar radiation than north. The olive trees were all rain-feed. These olives had a black surface color and they were collected from 12 trees of both the shady and the sunny area.
Oil extraction was performed using an Abencor labo- ratory oil mill (Comercial Abengoa SA, Spain) equipped with a hammer mill, a thermobeater, and a paste centrifuge. The extraction was carried out at 28 °C with kneading for 30 min. The oily must was decanted and ltered before analysis. Extraction was run in duplicate.
Individual Quanti cation of Phenolic Compounds in Oils
They were extracted from the oil with N,N-dimethylfor- mamide (DMF) [11]. Brie y, 0.6 g of oil was extracted with 3 × 0.6 mL of DMF; the extract was then washed with hexane, and N2 was bubbled into the DMF extract to eliminate residual hexane. Finally, the extract was ltered through a 0.22 μm pore size nylon lter and injected into the chromatograph.
The chromatographic system consisted of a Waters 717 plus autosampler, a Waters 600 pump, and a Waters heater module (Waters Inc., Mildford, MA, USA). A Spherisorb ODS-2 (5 μm, 25 cm × 4.6 mm i.d., Waters Inc.) column was used. Separation was achieved using an elution gradient with an initial composition of 90 % water (pH adjusted to 3.0 with phosphoric acid) and 10 % methanol [11]. The concentration of the latter solvent was increased to 30 % over 10 min and maintained for 20 min. Subsequently, the methanol percentage was raised to 40 % over 10 min, maintained for 5 min, and then increased to 50 %. Finally, the methanol percentage was increased to 60, 70, and 100 % in 5 min periods. Initial conditions were reached in 10 min. A ow of 1 mL/ min and a temperature of 35 °C were used in all of the analyses. A Waters 996 diode array detector and a Jasco FP-920 uorescence detector (Jasco, Tokyo, Japan) were connected in series. Hydroxytyrosol, hydroxytyrosol glycol and tyrosol were purchased from Sigma-Aldrich (St. Louis, MO, USA), apigenin and luteolin from Extrasynthese (Genay, France), and the rest of standards were obtained by semipreparative HPLC following a similar conditions as described above [11].
Total Contents of Hydroxytyrosol and Tyrosol in Oils
They were analyzed following the method described else- where [12]. Olive oil (2.5 g) and 2 M HCl (50 mL) were put into a 100 mL glass bottle that was closed with a polypropylene cap. The mixture was vigorously homogenized by agitation at 400 rpm in an orbital shaking incubator model WY-200 for 5 h (Comecta SA, Barcelona, Spain). Experiments were run at 25 °C. Finally, 2 mL of the aqueous phase was removed by a plastic pipet, ltered through a 0.22 μm pore size nylon lter, and injected into the chromatograph. The chromatographic system was the same as noted above, except the gradient program of solvents that was modifed, the washing period starting at 20 min from injection. Chromatograms were recorded at 280 nm and quanti cation was made using external calibration with standards (hydroxytyrosol and tyrosol) purchased from Sigma-Aldrich (St. Louis, MO, USA).
Squalene
Hydrocarbons were obtained by adsorption chromatography following the method described elsewhere [13]. GC was performed by using an Agilent 6890A chromatograph equipped with a cold on-column injector with oven-track system and a ameionization detector. A HP-5 column (5 % diphenyl/95 % dimethyl polysiloxane, length 15 m, 0.32 mm i.d. and 0.1 μm lm thickness; Agilent Tech.) was used. Hydrogen (140 kPa inlet pressure) was used as carrier gas and nitrogen as makeup gas. The oven temperature was held at 80 °C for 5 min and then increased at 45 °C/min to 120 °C and then at 5 °C/min to 310 °C where it was held for 7 min. The detector temperature was 350 °C. Concentration of hydrocarbons was obtained comparing the total area and the squalene internal standard area.
Tocopherols
They were determined by HPLC with fluorescence detection (excitation at 290 nm and emission at 330 nm), following IUPAC Standard Method 2.432 [14]. The column was a Lichrosorb Si 60 packed with silica (5 μm particle size) (Merck, Darmstadt, Germany). The mobile phase was n-hexane/isopropanol (99:1, v/v) with a ow rate of 1 mL/ min.
Fatty Acids and Sterols
They were measured according to European Community Regulation 702/2007 [15].
Statistical Analysis
Statistica software version 7.0 was used for data processing (Statistica for Windows, Tulsa, OK, USA). A comparison among mean variables was made by Duncan ́s multiple- range tests, and the differences were considered signi cant when p < 0.05.
Results and Discussion
Fatty Acids
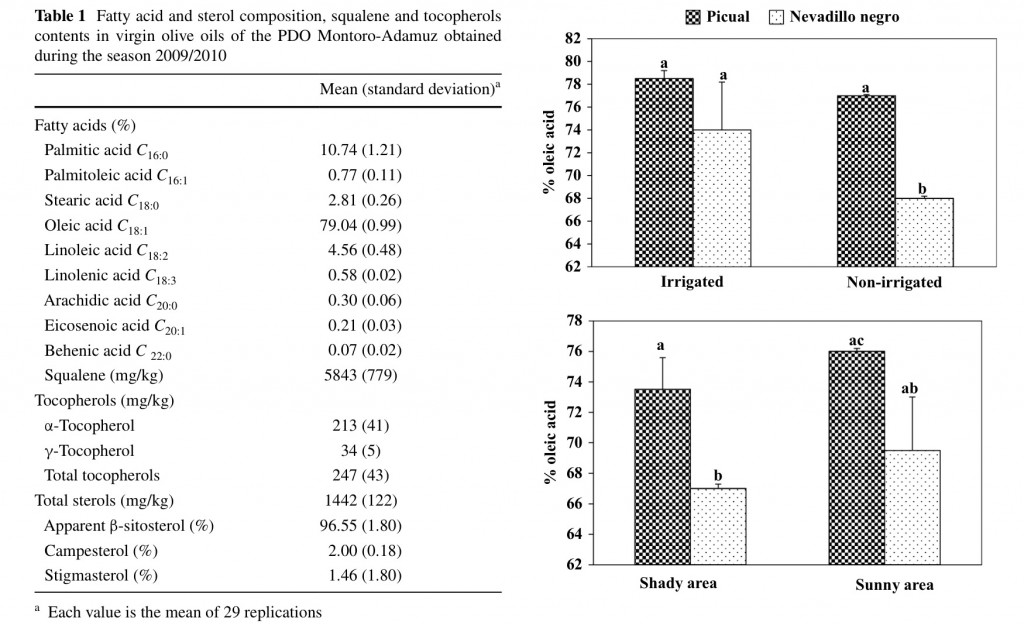
All the analyzed samples showed fatty acid content within the range required by European Regulation [15] (Table 1), being oleic acid the major fatty acid (ca. 79 %), which has been previously observed for VOO obtained from the Picual cultivar [16]. The EU regulation establishes a range for this monounsaturated acid between 55 and 83 % thereby the VOO of the PDO Montoro-Adamuz are included close to the highest level.
Figure 1 shows the effects of two agronomic factors on the content of oleic acid in these VOO. First, it must be noted that oils obtained from olives of the Nevadillo negro cultivar had a lower percentage of oleic acid than those of the Picual cultivar. However, the percentage of Nevadillo negro oil into the total fat of VOO of the PDO Montoro-Adamuz is lower than 10 % [10]. Otherwise, oils from olives cultivated under non-irrigation trees presented a lower percentage of oleic acid for both cultivars Picual and Nevadillo negro, which is in agreement with previous studies [17]. It is known that reduced growth temperatures increase membrane lipid unsaturation in order to maintain membrane uidity at low temperature, and several researchers have found higher percentage of oleic acid in oils obtained from cooler than warmer climate conditions of two different geographical areas [18]. In our study, oils from sunny areas trended to contain a higher percentage
Table 1 Fatty acid and sterol composition, squalene and tocopherols contents in virgin olive oils of the PDO Montoro-Adamuz obtained during the season 2009/2010
Fig. 1 Influence of agronomic conditions on the content in oleic acid
of oils obtained at laboratory scale of the Picual and Nevadillo negro
cultivar. Bars mean the standard deviation of two samples. Different
letters mean significant differences according to a Duncan´s multiple
range test (P < 0.05)
of oleic acid but it was not statistically signi cant (Fig. 1), thereby other agronomic conditions might influence to a large extent on the content of this fatty acid.
Squalene
There are no limits for this bioactive substance in the international regulations on olive oil but this compound has been found in VOO in a wide range from 800 to 12,000 mg/ kg [13]. Squalene values for Greek oils have been recorded between 2000 and 6500 mg/kg [19], and between 900 and 8700 mg/kg for Italian oils [20].
VOO of the PDO Montoro-Adamuz showed a mean squalene concentration of 5800 mg/kg, which is in accordance with previous data reported on Picual oils [21]. Like many other minor substances present in VOO, the concentration of squalene depends on olive cultivar, among other variables. The Nevadillo negro oils tended to possess a higher amount of this substance than those of the Picual cultivar (Fig. 2). Otherwise, the concentration of squalene was slightly in uenced by the irrigation regime of the olive trees, which is in disagreement with a previous work [22] that found a consistently lower content of squalene in oils from trees receiving the lowest irrigation level. In contrast, the location of the olive tree in the same orchard had a great in uence on the concentration of squalene in oils because both Picual and Nevadillo negro oils from fruit cultivated in the sunny area had a signi cant lower content than those from the shady area (Fig. 2). Several studies have shown that plants under environmental signals like high salinity, high UV-B levels and drought allocate squalene to produce triterpenes and sterols. Likewise, the content of squalene in both fruit and oil is in uenced by olive maturation [23], which is currently affected by sun radiation. Hence, there are some interactions between sun radiation level and other agronomic factors that could affect the content of oils in squalene.
Tocopherols
As expected, α-tocopherol was the major tocopherol in VOO of the PDO Montoro-Adamuz, followed by γ-tocopherol (Table 1). The mean value of total tocopherols was 247 mg/kg, which is in the average of the range previously reported for tocopherols in Spanish VOO (84- 463 mg/kg) [7], and in agreement with the content previ- ously found in Picual VOO [24]. It must be noted that the Nevadillo negro oils presented a higher concentration of these substances than the Picual (Fig. 3).
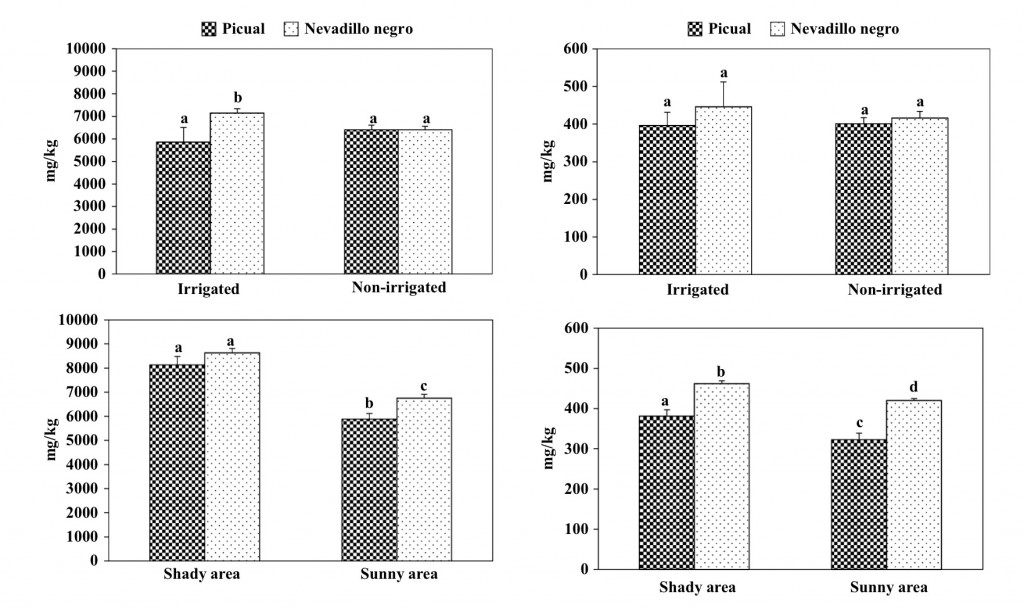 Fig. 2 Influence of agronomic conditions on the content in squalene of oils obtained at laboratory scale of the Picual and Nevadillo negro cultivar. Bars mean the standard deviation of two samples. Different letters mean significant differences according to a Duncan´s multiple range test (P < 0.05)
Fig. 2 Influence of agronomic conditions on the content in squalene of oils obtained at laboratory scale of the Picual and Nevadillo negro cultivar. Bars mean the standard deviation of two samples. Different letters mean significant differences according to a Duncan´s multiple range test (P < 0.05)
Fig. 3 Influence of agronomic conditions on the content in tocopherols
of oils obtained at laboratory scale of the Picual and Nevadillo negro cultivar. Bars mean the standard deviation of two samples. Different letters mean significant differences according to a Duncan’s multiple range test (P < 0.05)
Furthermore, no significant differences were observed in the concentration of tocopherols in VOO due to the irrigation regime. By contrast, the oils from olives cultivated in the sunny areas had a low content in these substances than those obtained from the shady area irrespective of the cultivar. It is assumed that the tocopherols content in VOO decreases as ripening progress, it is cultivar dependent, and the rainfall level affects its concentration, increasing with water-stress [7]. How- ever, no data are available on the effect of the sun radiation on olive trees during the year.
Sterols
A mean value of 1442 mg/kg oil was found for total sterols content in the VOO of the PDO Montoro-Adamuz (Table 1), which is in accordance with data previously reported for oils obtained from fruits of the Picual cultivar [24]. This is a value higher than the minimum of 1000 mg/kg oil established for authenticity of VOO by European legislation [15]. Although many factors can influence the content of VOO in sterols, their concentration currently range between 1000 and 2000 mg/kg, lower than data observed for other vegetable oils such as soybean, sun ower and rapeseed.
Phenolic Compounds
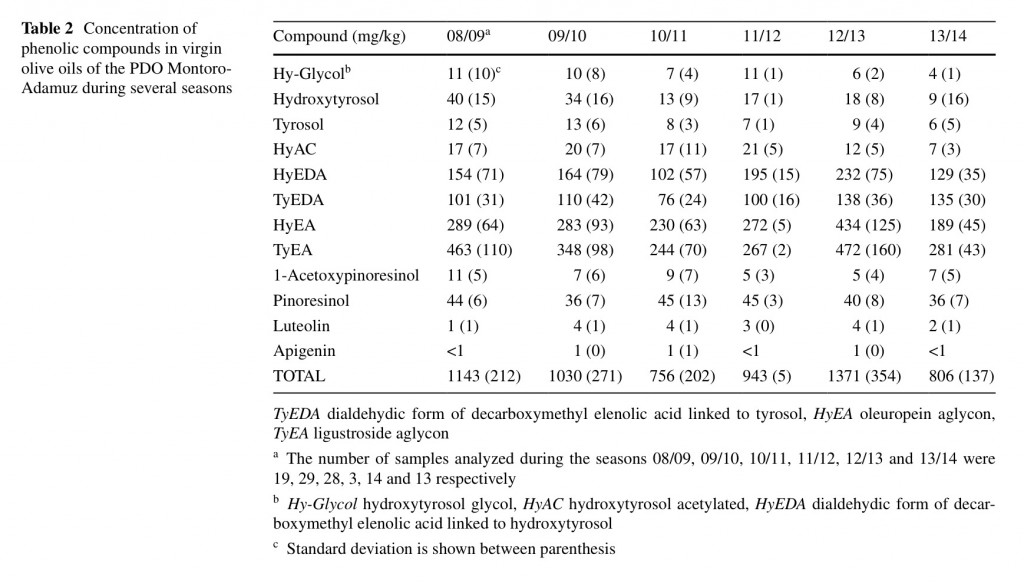
There are many studies that report the composition of VOO as in uenced by the growing area [25, 26], which has been mainly related to the rainfall level and altitude location of the olive trees. One of the main characteristics of the VOO of the PDO Montoro-Adamuz is their high content in phenolic compounds, which must be higher than 700 mg/ kg [10]. In Table 2, it is recorded the phenolic composition of these oils for the last six seasons. Previous works have reported the effect of the bearing cycle on the quality of olive oils [22] but few studies are available on the individual characterization of phenolic compounds in POD olive oils for many years. Obviously, there were differences among seasons but the total content was always higher than the limit of 700 mg/kg. Oils from the 2012/2013 season showed statistically higher concentration in total phenolic compounds than those of the 2008/2009, 2009/2010 and 201/2012 seasons, having those of the 2010/2011 and 2013/2014 season the lower statistical content. It must be noted that this is a very high concentration if it is compared with previous data from the literature [27].
In particular, these oils of Montoro-Adamuz are very rich in oleuropein and ligustroside aglycons (HyEA and TyEA), followed by the dialdehydic forms of decarboxymethyl elenolic acid linked to hydroxytyrosol (HyEDA) and tyrosol (TyEDA). The latter substance seems to be mainly responsible for the burning, pungent sensory notes in VOO [28], and it did not reach a higher concentration of 140 mg/kg in VOO of the Montoro-Adamuz, which could explain the non-extremely bitter sensation of these oils despite their high total phenolic content.
The phenolic profile of these oils also showed a significant presence of hydroxytyrosol, tyrosol, hydroxytyrosol acetylated, hydroxytyrosol glycol and, to a lesser extent, the avonoids luteolin and apigenin. Because of the low content in the lignan 1-acetoxypinoresinol, the percentage of Picual oil in the Montoro-Adamuz oil must be high since this substance has been proposed as a biomarker of Picual oils [11]. Table 3 shows the phenolic pro le of oils obtained from either Picual or Nevadillo negro fruit. It can be observed a big difference between these two oils, particularly the concentration of 1-acetoxypinoresinol was much lower in Picual oils than Nevadillo negro, whereas the opposite was found for the other lignan pinoresinol. These data corroborate the great contribution of the Picual cultivar to the characteristics of the oils of the PDO Mon- toro-Adamuz. Furthermore, the Nevadillo negro oil had a higher total phenolic content than the Picual oil, taking into consideration that all fruit were harvested in the same orchard.
 Fruits were cultivated in the same orchard and trees were not irrigated.
Fruits were cultivated in the same orchard and trees were not irrigated.
Values are the mean of duplicates
a Standard deviation is shown between parenthesis. See Table 2 for
compounds identification
Nevadillo negro oils were also richer in phenolic com- pounds irrespective of the irrigation regime or sun exposition level of the eld (Fig. 4). Likewise, total phenols were higher in oils from non-irrigated trees of the Nevadillo negro cultivar but the opposite behavior was found for the Picual cultivar. Previous works have shown that concentration of phenolic compounds decreases in VOO with increasing water irrigation of the olive trees [17, 29] but contradictory data has also been reported [30]. In fact, Moriana et al. [31] has proposed that the effect of irriga- tion on phenolic concentration takes place all year round and not just during the oil accumulation phase, which is the period when most growers irrigate their olive trees.
In our experiments, it was tested the effect of the sun exposition level of the olive trees on the phenolic content of the VOO Montoro-Adamuz (Fig. 4). Both Picual and Nevadillo negro oils obtained from a sunny area exhibited higher phenolic concentration that those of the shady area. Olives grown at high altitude give rise to oils with high concentration in phenolic compounds [24], and this effect can be related to the climatic conditions but also with a higher heat accumulation. Hence, it is reasonable to nd a high concentration of phenolic compounds in VOO obtained from olives cultivated in sunny areas.
As well as the individual quanti cation of the main phenolic compounds of the oils, it was measured the total concentrations of both hydroxytyrosol and tyrosol following the method proposed recently by Romero and Brenes (2012, 2014). There is not an of cial method to analyze phenolic compounds in olive oil because of several drawbacks but this new method allows the reliable determination of the total concentration of the two most important simple phenolic compounds present in olive oil. The average contents of hydroxytyrosol and tyrosol in VOO of the POD Montoro-Adamuz during the seasons 2008–2013 were 184 ± 60 and 186 ± 47 mg/kg respectively. These data are higher than most of those reported for many commercial Spanish VOO [12], and they con rm the richness of these oils in these bioactive substances.
Conclusions
Many variables contribute to the concentration of phenolic compounds in VOO such as cultivar, agronomic conditions, technological processing, irrigation and others. In the case of the VOO of the POD Montoro-Adamuz, it has been attributed to (1) the presence of the Nevadillo negro cultivar in the coupage of these oils, (2) the extreme agroclimatic conditions which cause physiological stress in the olive tree, and (3) the early harvesting of the fruit [10]. The results obtained in this work indicate that VOO of the POD Montoro-Adamuz are very rich in bioactive substances. They contain a high content in oleic acid, squalene, sterols and tocopherols but they are particularly rich in phenolic compounds, being their concentration higher than 700 mg/kg during the last six crop seasons. Besides, their total contents of hydroxytyrosol and tyrosol was higher than reported for many other VOO. The irrigation regime studied did not show a signi cant effect on the content of bioactive substances in these oils. By contrast, the level of sun radiation on olive trees exerted a great in uence on the concentration of these substances. Oils from trees cultivated in a sunny area had a higher percentage of oleic acid and concentration in phenolic compounds than those from shady areas. The opposite was detected for tocopherols and squalene which were more concentrated in oils from olives of the shady area. These results could contribute to make blended oils with speci c content in bioactive substances.
Acknowledgments The authors are grateful to Irene de la Rosa for
technical help. The authors also thank Antonio Terán and PDO Montoro-
Adamuz for providing samples and support.
References
1. Carrillo C, Cavia MD, Alonso-Torre SR (2012) Antitumor
effect of oleic acid; mechanisms of action; a review. Nutr Hosp
27:1860–1865
2. Varela LM, Ortega-Gomez A, Lopez S, Abia R, Muriana FJ, Bermudez
B (2013) The effects of dietary fatty acid on the postprandial
triglyceride-rich lipoprotein/apoB48 receptor axis in human
monocyte/macrophage cells. J Nutr Biochem 24:2031–2039
3. FDA (Food and Drug Administration, USA) (2004) Docket No.
2003Q-0559. Monounsaturated fatty acids from olive oil and
coronary heart disease
4. EC (European Commission) (2012) Regulation No 432/2012
establishing a list of permitted health claims made on foods. Off
J Eur Union L/136/1
5. Klingberg S, Ellegard L, Johansson I, Jansson JH, Hallmans G,
Winkvist A (2013) Dietary intake of naturally occurring plant
sterols is related to a lower risk of a first myocardial infarction
in men but not in women in Northern Sweden. J Nutr 143:1630–1635
6. Spanova M, Daum G (2011) Squalene-biochemistry, molecular
biology, process biotechnology, and applications. Eur J Lipid Sci
Technol 113:1299–1320
7. Beltrán G, Jiménez A, del Rio C, Sánchez S, Martínez L, Uceda
M, Aguilera MP (2010) Variability of vitamin E in virgin olive
oil by agronomical and genetic factors. J Food Comp Anal
23:633–639
8. Frankel EN (2011) Nutritional and biological properties of extra
virgin olive oil. J Agric Food Chem 59:785–792
9. García-González DL, Tena N, Aparicio R (2012) Describing the
chemical singularity of the Spanish protected designations of
origin for virgin olive oils in relation to oils from neighbouring
areas. Grasas Aceites 63:26–34
10. EC (European Commission) (2010). Publication of an applicant
pursuant to Article 6 (2) of Council Regulation (EC) No
510/2006 on the protection of geographical indications and designations
of origin for agricultural products and foodstuffs. Off J
Eur Union C 125/19
11. García A, Brenes M, Romero C, García P, Garrido A (2002) Use
of 1-acetoxypinoresinol to authenticate Picual olive oils. Int J
Food Sci Technol 37:615–625
12. Romero C, Brenes M (2012) Analysis of total contents of
hydroxytyrosol and tyrosol in olive oils. J Agric Food Chem
60:9017–9022
13. Lanzón A, Albi T, Cert A, Gracián J (1994) The hydrocarbon
fraction of virgin olive oil and changes resulting from refining. J
Am Oil Chem Soc 71:285–292
14. IUPAC (International Union of Pure and Applied Chemistry)
(1992) Standards methods for the analysis of oils, fats and derivatives.
1st Suppl. to 7th Edn., Pergamon Press, Oxford
15. EC (European Commission) (2007). Regulation EC/702/2007
amending Regulation EEC/2568/91 on the characteristics of
olive oil and olive-residue oil and on the relevant methods of
analysis. Off J Eur Union L-161/11-27
16. Beltrán G, del Rio C, Sánchez S, Martínez L (2004) Influence of
harvest date and crop yield on the fatty acid composition of virgin
olive oils from cv. Picual. J Agric Food Chem 52:3434–3440
17. Stefanoudaki E, Williams M, Chartzoulakis K, Harwood J (2009)
Effect of irrigation on quality attributes of olive oil. J Agric Food
Chem 57:7055–7408
18. Piravi-Vanak Z, Ghasemi JB, Ghavami M, Ezzatpanah H, Zolfonoun
E (2012) The influence of growing region on fatty acids
and sterol composition of Iranian olive oils by unsupervised
clustering methods. J Am Oil Chem Soc 89:371–378
19. Nenadis N, Tsimidou MZ (2002) Determination of squalene in
olive oil using fractional crystalization for simple preparation. J
Am Oil Chem Soc 79:257–259
20. De Leonardis A, Macciola V, De Felice M (1998) Rapid determination
of squalene in virgin olive oils using gas-liquid chromatography.
Ital J Food Sci 10:75–80
21. Samaniego-Sánchez C, Quesada-Granados JJ, López-García de
la Serrana H, López-Martínez MC (2010) ß-Carotene, squalene
and waxes determined by chromatography method in picual
extra virgin olive oil obtained by a new cold extraction system. J
Food Comp Anal 23:671–676
22. Ben-Gal A, Dag A, Basheer L, Yermiyahu U, Zipori I, Kerem
Z (2011) The influence of bearing cycles on olive oil quality
response to irrigation. J Agric Food Chem 59:11667–11675
23. Fernández-Cuesta A, León L, Velasco L, De la Rosa R (2013)
Changes in squalene and sterols associated with olive maturation.
Food Res 54:1885–1889
24. Aparicio R, Luna G (2002) Characterization of monovarietal virgin
olive oils. Eur J Lipid Sci Technol 104:614–627
25. Di Vaio C, Nocerino S, Paduano A, Sacchi R (2013) Influence
of some environmental factors on drupe maturation and olive oil
composition. J Sci Food Agric 93:1134–1139
26. Kesen S, Kelebek H, Selli S (2014) LC-ESI-MS characterization
of phenolic profiles Turkish olive oils as influenced by geographic
origin and harvest year. J Am Oil Chem Soc 91:385–394
27. García A, Brenes M, García P, Romero C, Garrido A (2003) Phenolic
content in commercial olive oils. Eur Food Res Technol
216:520–525
28. Andrewes P, Busch JLHC, Joode T, Groenewegen A, Alexandre
H (2003) Sensory properties of virgin olive oil polyphenols:
identification of deacetoxy-ligstroside aglycon as a key contributor
to pungency. J Agric Food Chem 51:1415–1420
29. Tovar MJ, Motilva MJ, Luna M, Girona J, Romero MP (2001)
Analytical characteristics of virgin olive oil from young trees
(Arbequina cultivar) growing under linear irrigation strategies. J
Am Oil Chem Soc 78:843–849
30. Servili M, Selvaggini R, Esposto S, Taticchi A, Montedoro GF,
Morozzi G (2004) Health and sensory properties of virgin olive
oil hydrophylic phenols: agronomic and technological aspects of
production that affect their occurrence in the oil. J Chromatogr
1054:113–127
31. Moriana A, Pérez-López D, Gómez-Rico A, Salvador M, Olmedilla
N, Ribas F, Fregapane G (2007) Irrigation scheduling for
traditional, low-density olive orchard: water relations and influence
on oil characteristics. Agric Water Manage 8:71–179